One example of an epigenetic change is DNA methylation — the addition of a methyl group, or a “chemical cap,” to part of the DNA molecule, which prevents certain genes from being expressed. Another example is the histone modification. Histones are proteins that DNA wraps around. The histone modification alters DNA accessibility and chromatin structure, thereby regulating patterns of gene expression. RNA-associated silencing is also another type of epigenetic mechanism. Epigenetic changes can be transmitted to daughter cells and also inherited. Some experiments have shown that some epigenetic changes can be reversed.
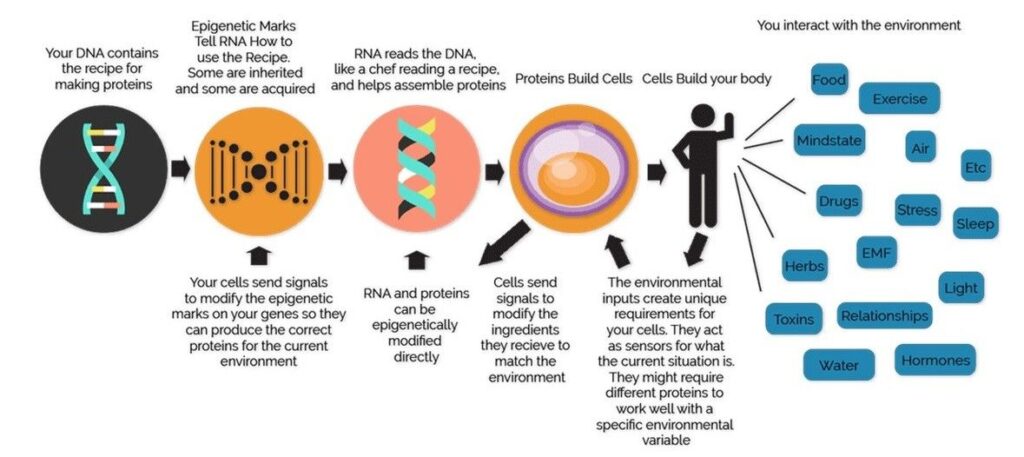
The different combinations of genes that are turned on or off is what makes each one of us unique. However, sometimes the expression of genes can get modified by certain external factors like nutrition, behavior, stress, physical activity, working habits, smoking, and alcohol consumption. Epigenetic changes, unlike DNA sequences which is the same in every cell, can occur in certain cells as a result of lifestyle and other environmental exposure (Figure 1). Epigenetic marks do not change DNA sequence, and therefore the entire genomic information or genotype, inherited from our parents remains untouched. However, due to epigenetic changes certain genes that are required to be switched off are erroneously turned on (activated) and vice-versa i.e., certain genes cannot be expressed due to lack of appropriate signals (silenced). Both these conditions may lead to serious health problems.
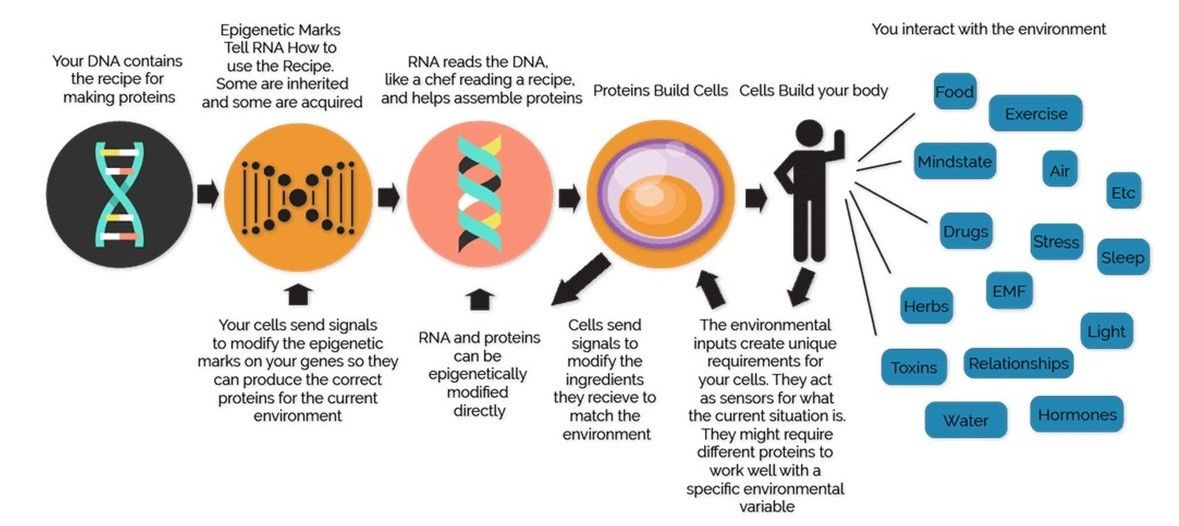
Figure 1: Epigenetics is the feedback loop between ours genes and the environment
An excess amount of the stress hormone, cortisol, in the body could impact epigenetic processes and boost one’s risk of experiencing psychological issues in the long run [2]. People with anxiety, post-traumatic stress, depression, and other stress-related disorders could be adjusting chemical tags on their DNA as a result of high cortisol exposure, which may even persist throughout the course of their lives or be passed on to their children.
It is well established that a balanced diet enhances life expectancy and helps to prevent or treat certain diseases, such as obesity, diabetes, cancer, and mental disorders. However, the biological mechanisms underlying these effects are not yet well understood. Some recently published studies in Nature Communications have explored how diet or compounds found in food can alter gene expression Programmes through epigenetic mechanisms, opening a new avenue in exploring therapies based on these mechanisms. Malnutrition in early life has directly and indirectly been linked to a number of disorders in adulthood, giving rise to the hypothesis of epigenetic memory [3].
Epigenetics involves genetic control by factors other than an individual’s DNA sequence [4]. Epigenetic changes can switch genes on or off and determine which proteins are transcribed. While epigenetic changes are required for normal development and health, they can also be responsible for some disease states. Disrupting any of the three systems that contribute to epigenetic alterations can cause abnormal activation or silencing of genes. Such disruptions have been associated with cancer, syndromes involving chromosomal instabilities, and mental retardation.
Genomic imprinting is the biological process that turns genes on and off to help control early mammalian development as the embryo and placenta grow. Although errors in genomic imprinting can cause profound developmental defects and severe disorders that lead to lifelong health problems, neither the mechanisms behind genomic imprinting nor the errors that cause errors in the process are well understood. Boston Children’s Hospital and Harvard Medical School scientists are working to remedy that shortcoming, and have identified a mechanism that regulates the process of gene imprinting in mice, including certain genes central to placental growth. If this mechanism is confirmed via further research in humans, it is likely to offer a range of epigenetic therapies for various developmental disorders such as autism.
As knowledge of the epigenome grows, we continue to learn more about how the substances we consume and the social situations we inhabit influence the way our genes are expressed. Scientists are already rethinking the way organisms evolve and how traits are passed on from parent to offspring. But it is still not known at what point will this knowledge begin to change the way we live? At what point will we be able to take a pill and block or unblock the right combination of genes to improve our quality of life? Following the completion of the Human Genome Project, the Human Epigenome Project is currently striving to map the scope of changes that can occur between genome and phenotype. Once finished, however, an epigenomic map could also prove useful in determining which individuals are at risk for certain diseases and encouraging the kind of lifestyle changes that can prevent the wrong genes from switching on or off.
References
1. Epigenetics. [Accessed from: https://en.wikipedia.org/wiki/Epigenetics].
2. Kirkpatrick B. Excess Stress Changes Marks on DNA and Could Epigenetically Harm Mental Health
July 5, 2017. [Assessed from: https://tinyurl.com/y5f7brnh].
3. Zhang Yi, Kutateladze TG. Diet and the epigenome. Nature Communications 2018; 9, Article number: 3375. [Assessed from: https://www.nature.com/articles/s41467-018-05778-1].
4. Simmons, D. Epigenetic influence and disease. Nature Education 2008; 1(1):6
5. Lant K. New Epigenetic Research Could Lead to Future Therapies for Developmental Disorders. 2017. [Assessed from: https://tinyurl.com/yyyf8qwm].
6. Lamb R. How Epigenetics Works. [Assessed from: https://tinyurl.com/yxf85gq3].
Source of Figure 1: http://flaxensaxon.blogspot.com/2018/10/epigenetics-sins-of-fathers.html
Dr. Sanjoy Kumar Pal is a Professor of Biology in Skyline University Nigeria. He has a PhD. in Animal Genetics from Indian Veterinary Research Institute, India.
You can join the conversation on Facebook @SkylineUniversityNG and on Twitter @SkylineUNigeria